Talk Overview
Differential Interference Contrast (sometimes known as Normarski microscopy) is a variation of polarization microscopy which generates a high contrast “shadow” image of a specimen. The mechanism of the DIC (Wollaston) prisms is discussed along with how to generate optimal contrast.
Questions
- A differential interference contrast microscope consists of a polarizer and analyzer and a single DIC prism located above the objective. True or False
- Which major feature of a DIC image is incorrect?
- In the direction of contrast, one edge of an object is bright and the other is dark
- Contrast is directional; orientations of the specimen that achieve minimal and maximal contrast occur with a rotation of 90 degrees (orthogonal).
- The center of a large uniform refractive index specimen will appear much brighter than the background.
- Each point is represented by two overlapping Airy disks; separation is typically ½ to 2/3rd of the Airy disk diameter
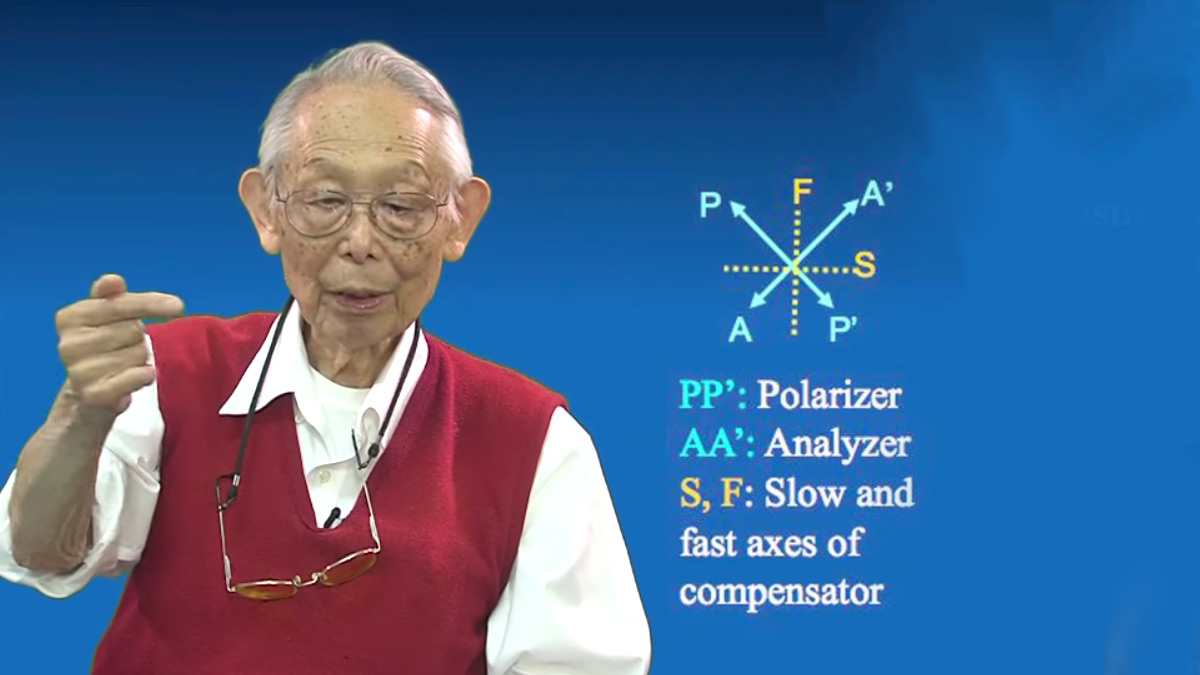
- The Wollaston Prism:
- Is located at the front plane of the condenser lens
- has its fast and slow axes oriented 45 degrees to the polarizer
- Resolves the plane polarized light into two orthogonal beams that traverse the specimen.
- Is used to change the wavelength of light
- A, B, C
- A, B, C, D
- A compensator in DIC microscopy:
- compensates for loss of light traveling through the DIC prisms
- Increases the brightness of the background and generates black and white contrast
- Extinguishes light that is phase shifted by the specimen
- Protects the camera from excess illumination
- Is usually located in the illumination path before the objective.
Answers
View AnswersSpeaker Bio
Ted Salmon

Ted Salmon is a Distinguished Professor in the Biology Department at the University of North Carolina. His lab has pioneered techniques in video and digital imaging to study the assembly of spindle microtubules and the segregation of chromosomes during mitosis. Continue Reading






Leave a Reply