Talk Overview
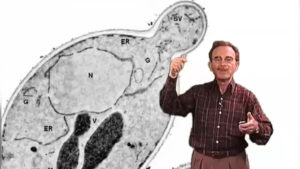
Protein secretion is executed by a cellular pathway involving the delivery of membrane and soluble secretory proteins in vesicles that capture newly-synthesized proteins assembled in the endoplasmic reticulum (ER) and sorted in the Golgi apparatus. Vesicles fuse with the plasma membrane resulting in the discharge of soluble molecules to the cell exterior and integration of vesicle membrane proteins and lipids in the cell surface. Baker’s yeast cells grow by vesicle fusion and secretion at the tip of the daughter bud. A genetic dissection of this process was performed with temperature sensitive conditional mutants blocked at one of several stations in the secretory pathway.
Secretion mutants that block protein exit from the endoplasmic reticulum define genes involved in the formation, targeting and fusion of a small vesicle intermediate. SEC genes corresponding to the mutants defective in vesicle budding define the cytoplasmic machinery responsible for transport vesicle morphogenesis. A biochemical reaction that reproduces ER vesicle budding was reconstituted with gently-broken yeast cells and pure recombinant Sec proteins required in vivo for this budding event. The Sec proteins assemble on the ER membrane in the presence of GTP which activates a small GTPase, Sar1, initiating the formation of a coat protein complex called COPII.
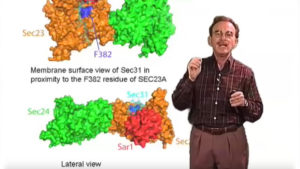
Human COPII genes are duplicated and some may have evolved specialized functions. Two rare human diseases affect the activity of one of two copies of Sar1 and the Sec23A subunit of the COPII coat. Anderson’s disease results in the failure of enterocytes of the absorptive epithelium to secrete large lipoprotein particles called chylomicrons. Point mutations in one of two copies of Sar1 results in the accumulation of chylomicrons in the ER. CLSD, a rare craniofacial disorder likely due to the selective failure of secretion of certain connective tissue proteins such as collagen, is caused by a conservative amino acid substitution in Sec23A that blocks completion of COPII coat assembly.






Leave a Reply